The cities that bacteria build
Bacteria build citadels that inspire architects and save the environment.
In nature, bacteria live in complex community structures known as biofilms. Living in biofilms provide significant benefits to bacteria. For example, biofilms protect their residents from environmental assaults, and improve their attachment to many different surfaces. However, biofilms play an important role in resistance to antibiotics (1, 2). The resident bacteria in a biofilm can be up to 1,000 times more resistant to antibiotics than free-living bacteria (1). The mechanisms behind this resistance are still poorly understood.
Bacterial biofilms can have deadly effects, such as those which are associated with many persistent and chronic bacterial infections, such as endocarditis, chronic lung infections in Cystic Fibrosis patients, and infections produced by medical devices (3). The current approaches to control biofilm infections involve the development of therapeutic agents that either prevent the formation of, or promote the detachment of biofilms (3).
Until recently, it was thought that bacteria form complex architectures exclusively via organic self-produced extracellular matrix. However, we have recently shown in our lab that microbes also construct communities atop a robust interior layer of calcium carbonate. This so-called “localized biomineralization” occurs during biofilm development in two distinct model organisms (specifically Bacillus subtilis, related to the human pathogen Bacillus cereus, and Mycobacterium smegmatis,related to the human pathogen Mycobacterium tuberculosis) (4).We identified bacterial genes and environmental triggers that contribute to this mineralization process. Our results indicate that this mineral matrix strengthens the biofilm integrity and serves as a frame to support larger bacterial populations. Also, these minerals can also function to protect bacteria from antibiotics.
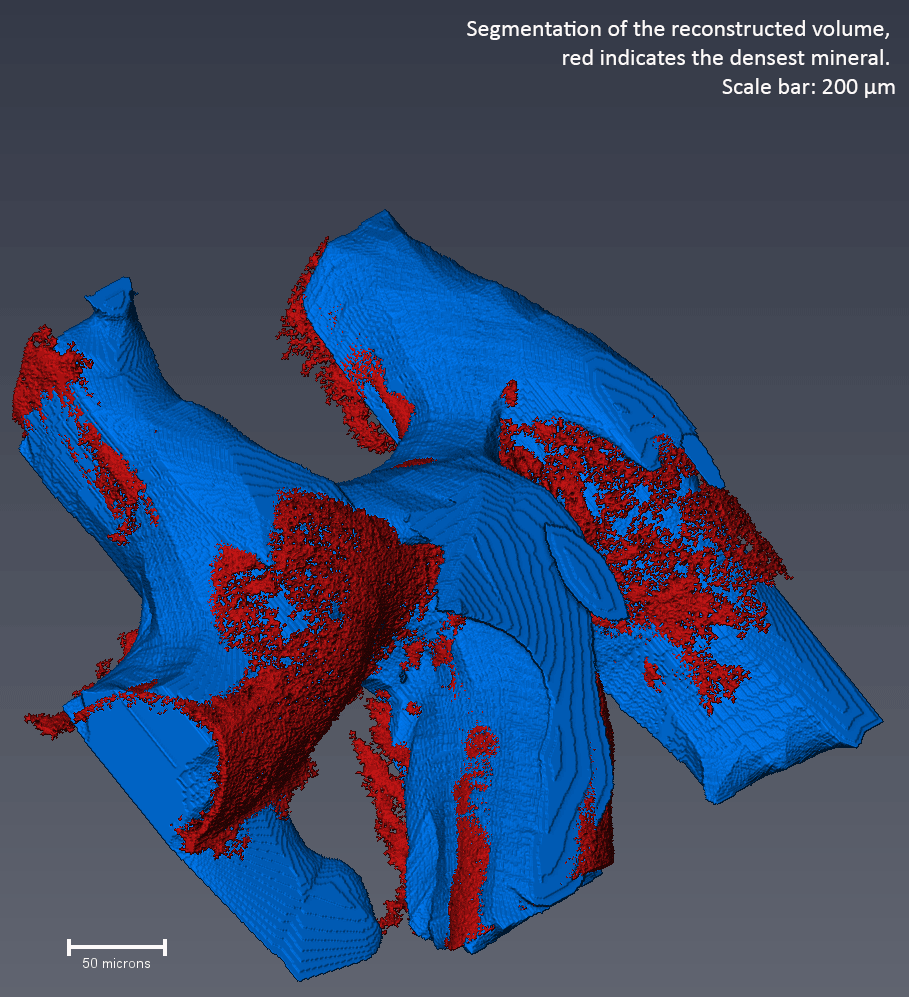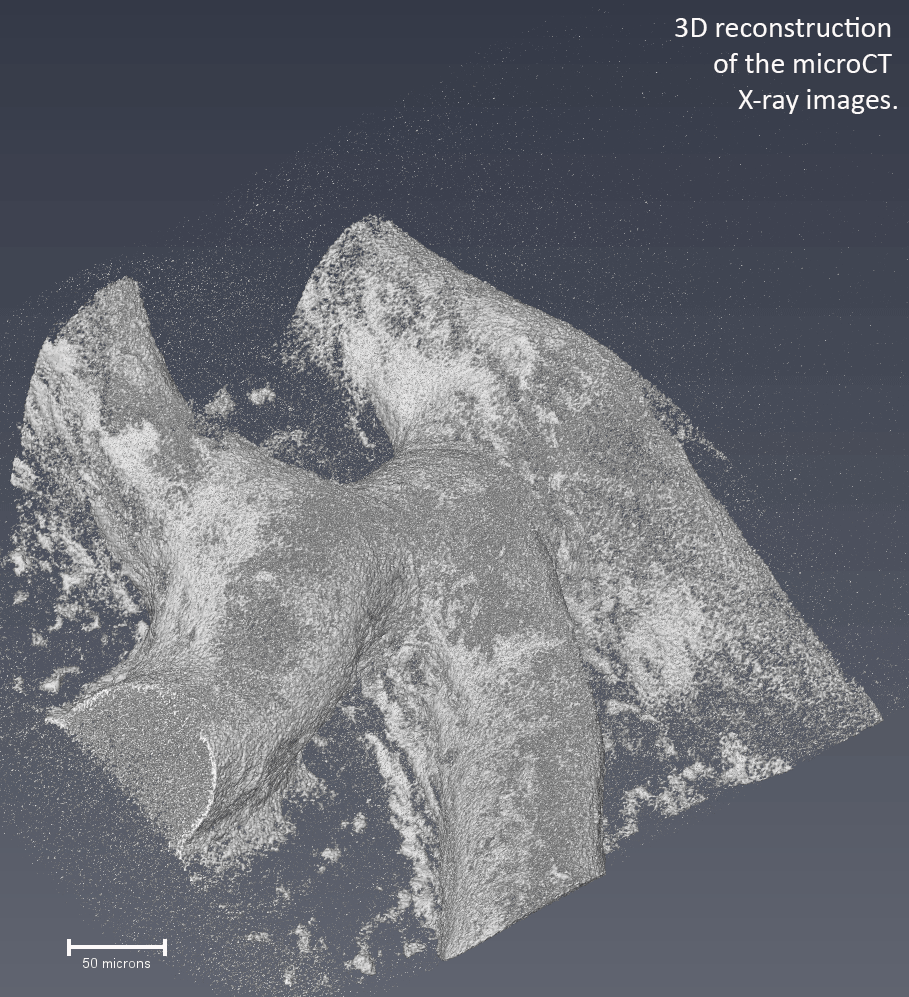
To study the contribution of minerals to the antibiotic resistance of biofilms, we developed a high-resolution µCT technique (X-ray) to visualize the mineralized areas within intact bacterial biofilms. We found that the calcite minerals form a barrier that shelters the inner cells of the biofilm colony. We also showed that blocking urease (a key enzyme in biomineralization) can hinder the formation of mineralized barriers and increase biofilm permeability5. In the clinic, this technology can be used to predict how permeable biofilms are to treatment. Also, biomineralization enzymes are attractive and novel therapeutic targets for highly resistant infections.
In his text ‘On Architecture’, the Roman architecture Vitruvius suggested that architecture is an imitation of nature. We began to wonder if and how the mineralized architecture in bacterial communities could be applied to the development of sustainable construction of actual buildings. How can we control the structure and function of mineralized products in this macro setting? This important question was partly answered by demonstrating that bacteria first secrete different organic materials that serve as a template, and then shape the calcium carbonate crystals formed by the bacterial cells. This provides a proof-of-concept for using of bacteria in designing rigid construction materials and altering crystal morphology and function. In the future, we could use select members of the soil microbiome to change the current strategies of architecture and construction. Perhaps genetically engineered bacterial biofilm communities, enhanced by synthetic circuits, could be used to construct environmentally sustainable buildings, and maybe even to sequester carbon dioxide, slowing down climate change in the long run.
© Ilana Kolodkin
References:
- Bryers, J. D. Medical biofilms. Biotechnology and bioengineering 100, 1-18, doi:10.1002/bit.21838 (2008).
- Hill, D. et al. Antibiotic susceptabilities of Pseudomonas aeruginosa isolates derived from patients with cystic fibrosis under aerobic, anaerobic, and biofilm conditions. Journal of clinical microbiology 43, 5085-5090, doi:10.1128/JCM.43.10.5085-5090.2005 (2005).
- Costerton, J. W., Stewart, P. S. & Greenberg, E. P. Bacterial biofilms: a common cause of persistent infections. Science 284, 1318-1322 (1999)
- Oppenheimer-Shaanan, Y. et al. Spatio-temporal assembly of functional mineral scaffolds within microbial biofilms. NPJ biofilms and microbiomes 2, 15031, doi:10.1038/npjbiofilms.2015.31 (2016)
- Keren-Paz, A., Brumfeld, V., Oppenheimer-Shaanan, Y. & Kolodkin-Gal, I. Micro-CT X-ray imaging exposes structured diffusion barriers within biofilms. NPJ biofilms and microbiomes4, 8, doi:10.1038/s41522-018-0051-8 (2018).

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.